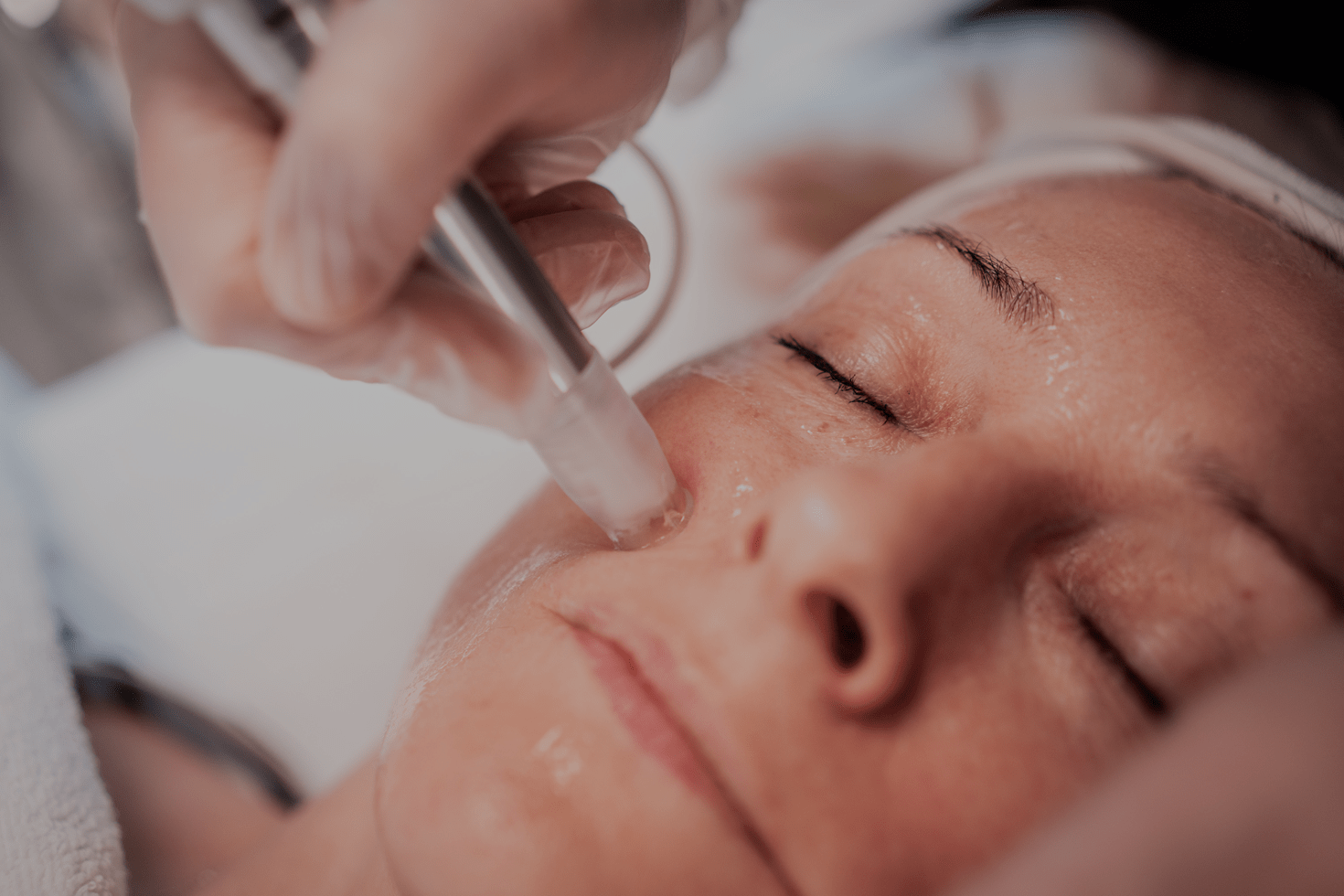
Highlights
Empowering professionals worldwide with our microneedling solutions.
25
Years
In Business
50
Countries
available
40,622
Satisfied
Customers
Featured Products
Medical-technical devices for aesthetic and medical treatments.
Diverse Treatment Areas
Unlocking the potential of microneedling for multiple indications.

Our Skincare Journey
Step into a realm where artistry meets medical precision, as we rewrite the story of skincare innovation and empower professionals to redefine beauty.
Treatment Tutorials
Watch our step-by-step tutorials and perfect your skin care routine for a healthier skin.